Who is eligible to undergo this treatment?
Individuals who are at least 18 years old with moderate to severe OSA (AHI between 15-100 events/hour), and with a BMI of 40 or less. It is also indicated for paediatric patients between 13 to 18 years old with Down syndrome who have moderate to severe OSA (AHI between 10 to 50). Patients who are unable to tolerate CPAP are usually recommended for this treatment. To assess eligibility, a sedated patient undergoes a drug-induced sleep endoscopy (DISE), a quick procedure where an ENT (ear, nose and throat) doctor uses a flexible nasoendoscope (camera) to assess how the airway collapses.
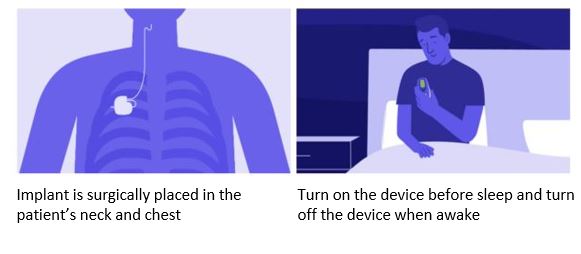
What can I expect during the surgery?
The surgical procedure typically lasts approximately two hours and is conducted under general anaesthesia. Two incisions will be made—one in the chest and another in the neck. These incisions will be covered with a dressing, which can be removed after two days.
What can I expect after the surgery?
Patients generally do not experience significant pain, although some soreness may be expected. Patients can return home on the same day of the surgery. After the surgery, patients can speak, swallow and shower as per usual and resume their regular diet. However, activities such as lifting heavy weights or vigorous exercises, especially those involving the upper body, should be avoided to allow the wounds sufficient time to heal.
What are the potential side effects?
The safety profile of this device has demonstrated excellence in numerous studies and real-world data. Non-serious side effects may include discomfort from the stimulation, temporary tongue weakness and wound swelling/infection. Device-related side effects, such as device migration or failure, are rare.